Toxo Igm: Kháng Thể Toxoplasma Gondii
Liên hệ
Hà Nội
Toxo IgM (IgM antibodies to Toxoplasma gondii)
Hóa chất có thể được sử dụng trên máy: Elecsys 2010, Modular analytics E170, cobas e 411, cobas e 601
Intended use
Immunoassay for the in vitro qualitative determination of IgM antibodies to Toxoplasma gondii in human serum and plasma.
The electrochemiluminescence immunoassay “ECLIA” is intended for use on Elecsys and cobas e immunoassay analyzers.
Summary
Toxoplasmosis is a common infection caused by the protozoan parasite Toxoplasma gondii.
The infection is mainly acquired by ingestion of food or water that is contaminated by mature oocysts shed by cats or by undercooked
meat containing tissue cysts.
Primary, acute infection which is mostly mild or even asymptomatic in healthy individuals, is followed by a latent infection which usually persists for life. However, reactivation of a latent Toxoplasma infection as a result of immunosuppression (e.g. in organ transplant recipients, AIDS patients)
is frequently associated with meningoencephalitis.
Primary maternal Toxoplasma infection occurring during pregnancy may lead to severe damage of the fetus as the parasite can be transmitted across the placenta. The majority of infants with congenital infection do not present clinical symptoms at birth but may develop severe sequelae later in life like mental and psychomotor retardation, chorioretinitis and hearing loss.
The fetal infection rate increases with gestational age. However, the risk of severe clinical manifestations is higher in case of early maternal infection.
Early drug therapy in acute infection during pregnancy can prevent congenital damage or ameliorate the severity of clinical manifestations.
The diagnosis of Toxoplasma infection is most commonly made by the detection of anti-Toxoplasma-specific IgG and IgM antibodies.
The detection of Toxo IgM antibodies is presumptive of an acute, recent or reactivated Toxoplasma infection.
The determination of IgG antibodies to Toxoplasma gondii is used to assess the serological status to T. gondii and is indicative of an acute or latent infection.
The diagnosis of the acute acquired infection during pregnancy is established by a seroconversion or a significant rise in antibody titers (IgG and/or IgM) in serial samples.
Test principle
µ-Capture test principle. Total duration of assay: 18 minutes.
• 1st incubation: 10 µL of sample are automatically prediluted 1:20 with Diluent Universal. T. gondii-specific recombinant antigen labeled with a ruthenium complex a is added. Anti-Toxo IgM antibodies present in the sample react with the ruthenium-labeled T. gondii-specific recombinant antigen.
• 2nd incubation: Biotinylated monoclonal h-IgM-specific antibodies and streptavidin-coated microparticles are added. The complex becomes bound to the solid phase via interaction of biotin and streptavidin.
• The reaction mixture is aspirated into the measuring cell where the microparticles are magnetically captured onto the surface of the electrode. Unbound substances are then removed with ProCell/ProCell M. Application of a voltage to the electrode then induces chemiluminescent emission which is measured by a photomultiplier.
• Results are determined automatically by the software by comparing the electrochemiluminescence signal obtained from the reaction product of the sample with the signal of the cutoff value previously obtained by Toxo IgM calibration.
a) Tris(2,2’-bipyridyl)ruthenium(II)-complex (Ru(bpy)2+3)
Reagents - working solutions
M Streptavidin-coated microparticles (transparent cap), 1 bottle, 6.5 mL: Streptavidin-coated microparticles 0.72 mg/mL; preservative.
R1 Toxoplasma-Ag~Ru(bpy)2+3 (gray cap), 1 bottle, 9 mL: Toxoplasma-antigen labeled with ruthenium complex > 1 mg/L; MESb buffer 50 mmol/L, pH 6.0; preservative.
R2 Anti-h-IgM-Ab~biotin (black cap), 1 bottle, 9 mL: Biotinylated monoclonal anti-h-IgM antibody (mouse) > 500 µg/L; HEPESc buffer 50 mmol/L, pH 7.2; preservative.
Cal1 Negative calibrator 1 (white cap), 2 bottles of 0.67 mL each: Human serum, negative for anti-Toxo IgM; preservative.
Cal2 Positive calibrator 2 (black cap), 2 bottles of 0.67 mL each: Anti-Toxo IgM (human) approx. 130 U/mL (Roche units) in human serum; preservative.
b) MES = 2-morpholino-ethane sulfonic acid
c) HEPES = [4-(2-hydroxyethyl)-piperazine]-ethane sulfonic acid
Precautions and warnings
For in vitro diagnostic use.
Exercise the normal precautions required for handling all laboratory reagents.
Disposal of all waste material should be in accordance with local guidelines.
Safety data sheet available for professional user on request.
All human material should be considered potentially infectious. All products derived from human blood (Cal1, Cal2) are prepared exclusively from the blood of donors tested individually and shown to be free from HBsAg and antibodies to HCV and HIV.
The serum containing anti-Toxo IgM (Cal2) was sterile filtrated.
The testing methods applied were FDA-approved or cleared in compliance with the European Directive 98/79/EC, Annex II, List A.
However, as no testing method can rule out the potential risk of infection with absolute certainty, the material should be handled with the same level of care as a patient specimen. In the event of exposure, the directives of the responsible health authorities should be followed.
The reagents may not be used after the stated expiration date.
Avoid foam formation in all reagents and sample types (specimens, calibrators, and controls).
Reagent handling
The reagents in the kit are ready for use and are supplied in bottles compatible with the system.
Elecsys 2010 and cobas e 411 analyzers: The calibrators should only be left on the analyzer during calibration at 20-25 °C. After use, close the bottles as soon as possible and store at 2-8 °C. Due to possible evaporation effects, not more than 5 calibration procedures per calibrator bottle set should be performed.
MODULAR ANALYTICS E170, cobas e 601 and cobas e 602 analyzers: Unless the entire volume is necessary for calibration on the analyzer, transfer aliquots of the ready-for-use calibrator into empty snap-cap bottles (CalSet Vials). Attach the supplied labels to these additional bottles. Store the aliquots at 2-8 °C for later use.
Perform only one calibration procedure per aliquot.
All information required for correct operation is read in from the respective reagent barcodes.
Storage and stability
Store at 2-8 °C.
Store the Elecsys Toxo IgM reagent kit upright in order to ensure complete availability of the microparticles during automatic mixing prior to use.
Stability: Stability of the reagent rackpack unopened at 2-8 °C up to the stated expiration date after opening at 2-8 °C 12 weeks on MODULAR ANALYTICS E170, Elecsys 2010 and cobas e 2 weeks or 12 weeks if stored alternately in the refrigerator and on the analyzers (up to 84 hours)
Stability of the calibrators
unopened at 2-8 °C up to the stated expiration date after opening at 2-8 °C 8 weeks on Elecsys 2010 and cobas e 411 at 20-25 °C up to 5 hours on MODULAR ANALYTICS E170, cobas e 601 and cobas e 602 use only once
Store the calibrators upright! Do not freeze. Ensure that no calibration solution is trapped in the opened snap-cap.
Specimen collection and preparation
Only the specimens listed below were tested in a sufficient number and found acceptable.
Serum collected using standard sampling tubes or tubes containing separating gel.
Li-heparin, K3-EDTA and Na-citrate plasma.
Criterion: Mean recovery of positive samples within 80-120 % of serum value.
Stable for 3 weeks at 2-8 °C, 3 days at 25 °C, 3 months at -20 °C.
The samples may be frozen 6 times.
The sample types listed were tested with a selection of sample collection tubes that were commercially available at the time of testing, i.e. not all available tubes of all manufacturers were tested. Sample collection systems from various manufacturers may contain differing materials which could affect the test results in some cases. When processing samples in primary tubes (sample collection systems), follow the instructions of the tube manufacturer.
Specimens should not be subsequently altered with additives (biocides, anti-oxidants or substances that could possibly change the pH of the sample) in order to avoid erroneous findings.
Pooled samples and other artificial material may have different effects on different assays and thus may lead to discrepant findings.
Centrifuge samples containing precipitates and frozen samples before performing the assay. Lyophilized samples, heat-inactivated samples and samples and controls stabilized with azide (up to 1 %) can be used.
Ensure the samples, calibrators, and controls are at ambient temperature (20-25 °C) before measurement.
Due to possible evaporation effects, samples and calibrators on the analyzers should be analyzed/measured within 2 hours.
Assay
For optimum performance of the assay follow the directions given in this document for the analyzer concerned. Refer to the appropriate operator’s manual for analyzer-specific assay instructions.
Resuspension of the microparticles takes place automatically prior to use.
Read in the test-specific parameters via the reagent barcode. If in exceptional cases the barcode cannot be read, enter the 15-digit sequence of numbers.
Bringthecooledreagents toapprox.20°C andplaceonthereagentdisk (20°C) of the analyzer. Avoid foam formation. The system automatically regulates the temperature of the reagents and the opening/closing of the bottles.
Place the calibrators in the sample zone. All the information necessary for calibrating the assay is automatically read into the analyzer. After calibration has been performed, store Cal1 and Cal2 at 2-8 °C or discard (MODULAR ANALYTICS E170, cobas e 601 and cobas e 602 analyzers).
Calibration
Traceability: This method has been standardized against a Roche standard. The units have been selected arbitrarily.
Calibration frequency: Calibration must be performed once per reagent lot using Elecsys Toxo IgM Cal1, Cal2, and fresh reagent (i.e. not more than 24 hours since the reagent kit was registered on the analyzer).
Renewed calibration is recommended as follows:
• after 1 month (28 days) when using the same reagent lot
• after 7 days (when using the same reagent kit on the analyzer)
• as required: e.g. quality control findings with PreciControl Toxo IgM outside the defined limits
• more frequently when this is required by pertinent regulations
Range for the electrochemiluminescence signals (counts) for the calibrators: Negative calibrator (Cal1): 400-2500 (Elecsys 2010, MODULAR ANALYTICS E170 and cobas e analyzers).
Positive calibrator (Cal2): 4500-35000 (Elecsys 2010, MODULAR ANALYTICS E170 and cobas e analyzers).
Quality control
For quality control, use PreciControl Toxo IgM.
Controls for the various concentration ranges should be run individually at least once every 24 hours when the test is in use, once per reagent kit, and following each calibration. The control intervals and limits should be adapted to each laboratory’s individual requirements. Values obtained should fall within the defined limits.
Each laboratory should establish corrective measures to be taken if values fall outside the defined limits.
If necessary, repeat the measurement of the samples concerned.
Follow the applicable government regulations and local guidelines for quality control.
Note:
For technical reasons re-assigned target values valid for a specific reagent and control lot combination only, must be entered manually on all analyzers (except for the cobas e 602 analyzer). Therefore, always consider the value sheet included in the rackpack or PreciControl kit to make sure that the correct target values are used.
When a new reagent or control lot is used, the analyzer will use the original values encoded in the control barcodes.
Limitations - interference
A negative Toxo IgM test result, also in combination with a positive Toxo IgG result, does not completely rule out the possibility of an acute infection with Toxoplasma gondii:
• Individuals at the early stage of acute infection may not exhibit detectable amounts of Toxo IgM antibodies. In some of these individuals an indeterminate or low positive result with the Elecsys Toxo IgG assay may be found and indicate an early acute infection. A second sample should be tested e.g. within 2 weeks. The detection of Toxo IgM and/or a significant increase of the Elecsys Toxo IgG antibody titer in the second sample supports the diagnosis of acute Toxoplasma infection.
• In some individuals Toxoplasma IgM-specific antibodies may revert to non-reactive levels within few weeks after infection with T. gondii.
The detection of IgM antibodies against T. gondii in a single sample is not sufficient to prove an acute Toxoplasma infection since elevated IgM antibody levels may persist even for years after initial infection.
Further tests or a combination of test methods should be done for clarification.
A significant increase of the Toxo IgG antibody titer from a first to a second sample taken e.g. within 2 weeks may support the diagnosis of acute Toxoplasma infection.
If a treatment is prescribed early enough, antibody production may not increase. IgG and IgM levels may remain low and can coexist for years.
Elecsys Toxo IgM results should be used in conjunction with Toxoplasma-specific IgG results, the patient’s medical history, clinical symptoms and other laboratory tests.
The results in HIV patients, in patients undergoing immunosuppressive therapy, or in patients with other disorders leading to immune suppression, should be interpreted with caution.
Specimens from neonates, cord blood, pretransplant patients or body fluids other than serum and plasma, such as urine, saliva or amniotic fluid have not been tested.
The assay is unaffected by icterus (bilirubin < 684 µmol/L or < 40 mg/dL), hemolysis (Hb < 1.24 mmol/L or < 2 g/dL), lipemia (Intralipid < 2000 mg/dL), and biotin (< 246 nmol/L or < 60 ng/mL).
Criterion: Mean recovery of positive samples within ± 20 % of serum value.
Samples should not be taken from patients receiving therapy with high biotin doses (i.e. > 5 mg/day) until at least 8 hours following the last biotin administration.
No interference was observed from rheumatoid factors up to a concentration of 3720 IU/mL.
The high-dose hook effect does not lead to false-negative results in the Elecsys Toxo IgM assay.
In vitro tests were performed on 18 commonly used pharmaceuticals and in addition on spiramycine, sulfadiazine, folic acid and pyrimethamine.
No interference with the assay was found.
As with many µ-capture assays an interference with unspecific IgM is observed. Increasing amounts of unspecific IgM may lead to a decrease in the recovery of positive samples with the Elecsys Toxo IgM assay.
In rare cases, interference due to extremely high titers of antibodies to immunological components, streptavidin or ruthenium can occur.
These effects are minimized by suitable test design.
For diagnostic purposes, the results should always be assessed in conjunction with the patient’s medical history, clinical examination and other findings.
Specific performance data
Representative performance data on the analyzers are given below.
Results obtained in individual laboratories may differ.
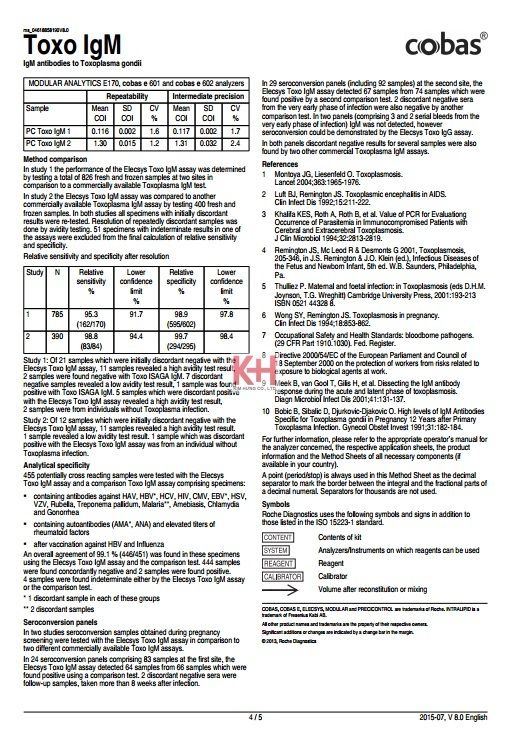
Precision
Precision was determined using Elecsys reagents, human sera, and controls (repeatability n = 21, intermediate precision n = 10); intermediate precision on MODULAR ANALYTICS E170 analyzer was determined in a modified protocol (EP5-A) of the CLSI (Clinical and Laboratory Standards Institute): 6 times daily for 10 days (n = 60).
http://kimhung.vn/vi/shops/Bang-chi-dau-xuong-Elecsys/Hoa-chat-mien-dich-Roche-1710/


Toxo IgM: Xét nghiệm kháng thể Toxoplasma gondii
Quý khách cần thêm thông tin nào khác vui lòng liên hệ :
NGUYEN THANH TUNG
Bác Sỹ
Kim Hung Co., Jsc
Trụ sở chính/Headquarters : 86 Le Duan Str., Hoan Kiem Dist., Hanoi, Vietnam
Tel/Fax: (+84-4) 3941 3887 / (+84-4) 3632 0598
Mobile : (+84) 912 775 249
Email : kimhung.sales@gmail.com
Web : http://www.kimhung.vn
Chi nhánh/Branch : P 1081 ,Tầng 18-VSBC Tower - 383B Cộng Hòa - P.13 - Q.Tân Bình - Tp. HCM
Tel/Fax: (+84-4) 6292 6913
Mobile : (+84) 963 889 249
Email : kimhung.sales@gmail.com
Web : http://www.kimhung.vn
HẾT HẠN
| Mã số : | 12205166 |
| Địa điểm : | Hồ Chí Minh |
| Hình thức : | Cần bán |
| Tình trạng : | Hàng mới |
| Hết hạn : | 09/10/2016 |
| Loại tin : | Thường |










Bình luận